· Blog · 120 min read
POWWeR Health Study Results
Screening and Enrollment flow Analysis Flow Reporting Tables Table 1. Expected and Actual Enrollment by month during the enrollment period Month,...
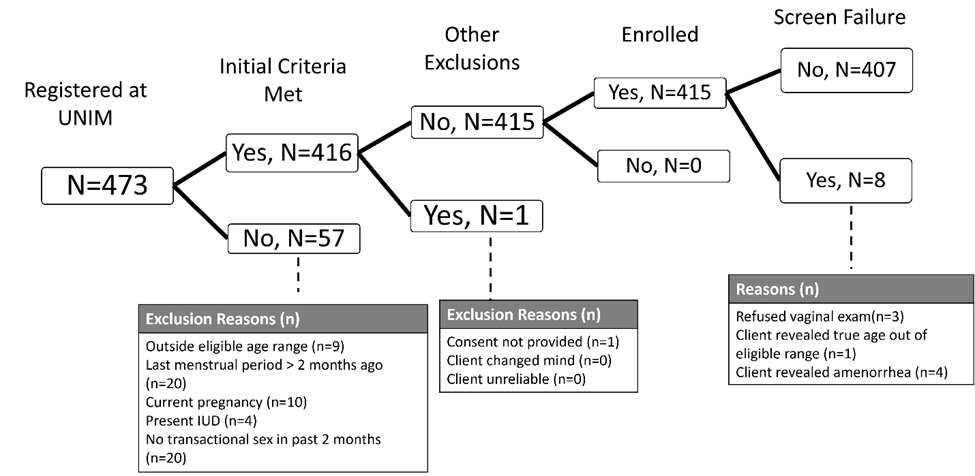
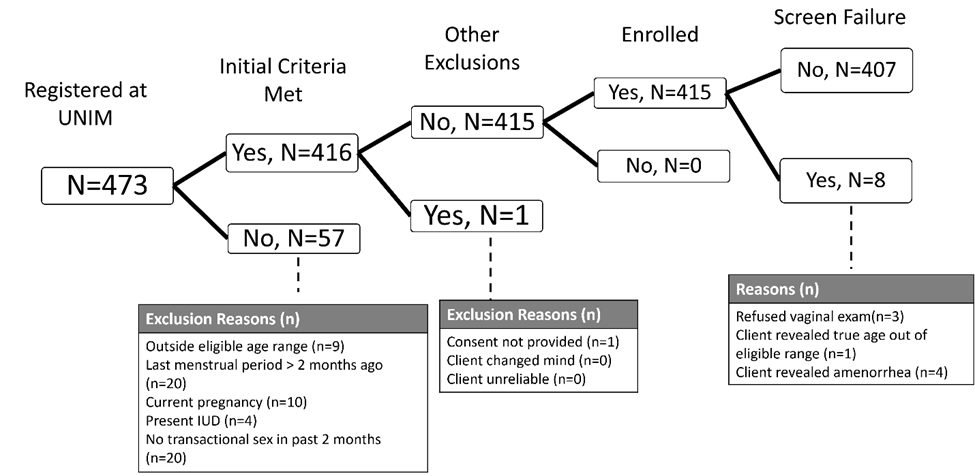
Screening and Enrollment flow
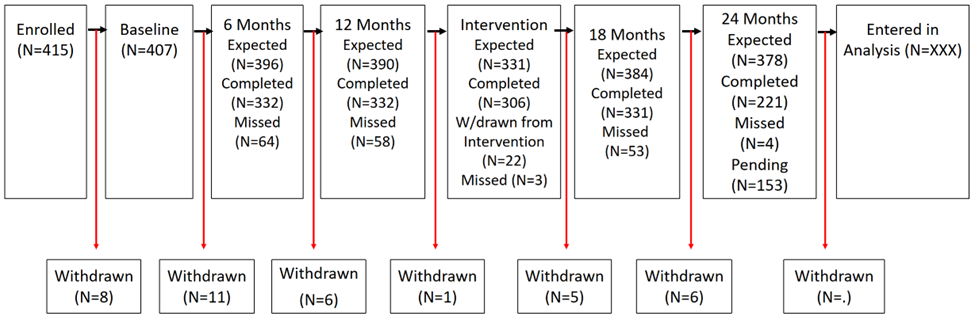
Analysis Flow
Reporting Tables
Table 1. Expected and Actual Enrollment by month during the enrollment period
|
Month, Dates |
Expected
Enrollment, N (%) |
Actual,
N (%) |
|
Month 1, Feb 9 - 28 |
67 |
41 |
|
Month 2, March 1 – 27 |
67 |
51 |
|
Month 3, April 3 - 28 |
67 |
18 |
|
Month 4, May 10-31 |
67 |
51 |
|
Month 5, June 1-30 |
67 |
50 |
|
Month 6, July 1-31 |
67 |
52 |
|
Month 7, Aug 1-30 |
- |
84 |
|
Month 8, Sept 1-30 |
- |
60 |
|
Total |
402 |
407 |
Table 2. Completed Study Forms
|
Database Forms |
Number
Completed |
||||
|
|
Baseline |
6 Months |
12Months |
18Months |
24Months |
|
Screening
& Eligibility |
473 |
X |
X |
X |
X |
|
Locator Form1 |
407 |
X |
X |
X |
X |
|
Behavioral
Survey |
407 |
332 |
332 |
331 |
221 |
|
Medical Exam Form |
407 |
332 |
332 |
331 |
221 |
|
Clinical
Diagnosis & Treatment Form |
407 |
3 |
332 |
6 |
221 |
|
Lab Requisition & Results |
407 |
326 |
332 |
331 |
221 |
|
HIV
C&T Form |
407 |
0 |
332 |
0 |
221 |
|
Withdrawal Form (prior to the stated
visit) |
|
11 |
6 |
6* |
6 |
|
Etiological
Diagnosis and Treatment Form |
147 |
95 |
167 |
115 |
90 |
|
WASH Assessment |
47 |
0 |
0 |
0 |
49 |
|
Total |
|
|
|
|
|
Table 3. Expected and Actual Study Visits by Time Point
|
Database Forms |
Expected |
Actual |
|
Initial Screen |
503 |
473 |
|
Baseline Enrollment |
402 |
407 |
|
6 Month Follow-Up (August 2023) |
41 |
52 |
|
6 Month Follow –Up (September) |
51 |
41 |
|
6 Month Follow – Up (October) |
18 |
32 |
|
6 Month Follow - Up (November) |
51 |
25 |
|
6 Month Follow – Up (December) |
50 |
14 |
|
6 Month Follow – Up (January 2024) |
52 |
66 |
|
6 Month Follow – Up (February 2024) |
84 |
40 |
|
6 Month Follow – Up (March 2024) |
60 |
54 |
|
6 Month Follow – Up (April 2024) |
14 |
8 |
|
12 Month Follow Up (January 2024) |
13 |
|
|
12 Month Follow UP (February 2024) |
52 |
51 |
|
12 Month Follow Up (March 2024) |
41 |
27 |
|
12 Month Follow Up (April 2024) |
32 |
36 |
|
12 Month Follow Up (May 2024) |
25 |
26 |
|
12 Month Follow Up (June 2024) |
14 |
21 |
|
12 Month Follow Up (July 2024) |
66 |
37 |
|
12 Month Follow Up (August 2024) |
40 |
56 |
|
12 Month Follow Up (September 2024) |
54 |
57 |
|
12 Month Follow Up (October 2024) |
8 |
4 |
|
12 Month Follow Up (November 2024) |
1 |
|
|
Intervention |
306 |
|
|
18 Month Follow Up (July) |
14 |
11 |
|
18 Month Follow Up (August) |
51 |
43 |
|
18 Month Follow up (September) |
27 |
44 |
|
18 Month Follow Up (October) |
36 |
9 |
|
18 Month Follow Up (November) |
26 |
40 |
|
18 Month Follow Up (December) |
21 |
13 |
|
18 Month Follow Up (January 2025) |
41 |
38 |
|
18 Month Follow Up (February 2025) |
67 |
55 |
|
18 Month Follow Up (March 2025) |
64 |
66 |
|
18 Month Follow Up (April 2025) |
16 |
12 |
|
18 Month Follow Up (May 2025) |
2 |
1 |
|
18 Month Follow Up (June 2025) |
1 |
|
|
24 Month Follow-Up |
322* |
|
|
24 Month Follow Up (January 2025) |
12 |
24 |
|
24 Month Follow Up (February 2025) |
41 |
46 |
|
24 Month Follow Up (March 2025) |
3 |
45 |
|
24 Month Follow Up (April 2025) |
3 |
8 |
|
24 Month Follow Up (May 2025) |
5 |
44 |
|
24 Month Follow Up (June 2025) |
129 |
54 |
|
24 Month Follow Up (July 2025) |
85 |
|
|
24 Month Follow Up (August 2025) |
||
|
24 Month Follow Up (September 2025) |
||
|
24 Month Follow Up (October 2025) |
|
|
|
24 Month Follow Up (November 2025) |
|
|
Table 4. Number of early withdrawals from the trial
|
Total
Early Withdrawal |
Withdrawn
from Study N
(%) |
Withdrawn
from Intervention but continuing in study |
|
Screen
failure: After consent, ineligible (gave incorrect birthdate (n=1), disclosed
amenorrhea (n=4), declined pelvic exam (n=2), died (n=1)) |
8 |
|
|
After
Baseline, before 6 months (no longer interested (n=2), relocated (n=8),
incarcerated (n=1)) |
11 |
|
|
After
6 Month Follow-Up, before 12 months (no longer interested (n=4),
relocated (n=2)) |
6 |
|
|
After
12 Month Follow-Up, before Intervention (co-enrolled in another study (n=1)) |
1 |
|
|
Completed
12 Month Follow-Up, and withdrawn by investigators from Intervention1 |
|
22 |
|
After
Completion of Intervention and before 18 Month Follow-Up (enrolled
in a conflicting study DoxyDot[GZ1] (n=1), inserted an IUD (n=2), no longer interested
(n=1), relocated (n=1) |
5 |
|
|
After
18 Month Follow-Up and before 24 Month Follow-Up -no longer interested (n=4),
relocated (n=1), died (n=1) |
6 |
|
Table 5. Number and Type of protocol deviations
|
Protocol Deviations |
N |
|
Issues with enrollment: |
0 |
|
Outside of Age Range |
0 |
|
Other exclusion/inclusion criterion
error |
0 |
|
Intervention
Delayed > 4 weeks1 |
6 reported to IRB |
|
Other: |
|
|
Extra specimens/tests taken |
0 |
|
Wrong appointment date2 Late
for 12M visit due to traveling, n=3: 27 days late, 14 days late, 20 days late Late
for 18M visit due to traveling, n=4: 4d late, 10d late, 2d late, 9d late Early for 12M visit, n=4: 5d early b/c
traveling to TZ and would miss visit Early for M24 Visit n=1: 15 days
early, Late
for M24 Visit n=1: Late for 2 days |
13 reported to IRB |
|
Serious adverse event not reported within 48 hours |
0 |
|
ID assigned to two clients but
corrected |
0 |
|
Appropriate form(s) not completed |
0 |
|
Appropriate specimens/tests not taken |
2 not reported |
|
Sample results delayed |
0 |
|
Impersonator took visit |
0 |
|
Wrong treatment given for BV |
85 – reported all to IRBs, NIH, and
DSMB |
|
Total |
106 |
Table 6. Baseline Characteristics
|
Baseline Characteristics |
N
(%) |
|
Age in years (median, IQR) |
27.3
(23.7-31.1) |
|
Ethnic
Group: |
|
|
Luo |
365
(89.7) |
|
Other |
42
(10.3) |
|
Education Level: |
|
|
< Secondary school |
241
(59.2) |
|
Secondary school or more |
166
(40.8) |
|
Employment |
|
|
Sex work only |
238
(58.5) |
|
Sex work + other |
169
(41.5) |
|
Has non-paying male partner (husband,
boyfriend, etc.) |
283
(69.5) |
Table 7. Baseline Vaginal Microbiome, STI and HIV, and Signs and Symptoms of Infection
|
Baseline Physical Characteristics |
N
(%) |
|
Laboratory
Results |
|
|
Herpes
Simplex Virus Type 2 seropositive (invalid,
n=3; missing n=1) |
253
(62.8) |
|
Composite STI |
90
(22.1) |
|
Trichomonas vaginalis (missing, n=2) |
30
(7.4) |
|
Neisseria gonorrhoeae
(missing, n=1) |
22
(5.4) |
|
Chlamydia trachomatis (missing, n=1) |
53
(13.0) |
|
Bacterial vaginosis (missing, n=2) |
171
(42.0) |
|
HIV
(missing, n=1) |
100 (24.6) |
|
Community State Type (CST) (missing,
n=1)
CST-I (L. crispatus dominated)
CST-II (L. gasseri dominated)
CST-III (L. iners dominated)
CST-IV (G. vaginalis dominated; mixed)
CST-V (L. jensenii dominated) |
31
(7.6) 1
(0.2) 118
(29.1) 255
(62.8) 1
(0.2) |
|
Physical Exam Results |
|
|
Vaginal or cervical discharge |
55
(13.5) |
|
Genital
ulcers or vesicles |
3
(0.9) |
|
Cervical motion tenderness or
friability |
3
(0.9) |
|
Adnexal
tenderness or mass |
3
(0.9) |
|
Cervical abnormalities: laceration,
excoriation, edema, erosion, abrasion, ecchymosis, tear, fissure |
1
(0.3) |
Table 8. Baseline Confounders
|
Baseline Confounders |
N
(%) |
|
HSV-2 Serostatus |
253
(62.8) |
|
HIV
Status |
100
(24.6) |
|
Employment
Sex work only
Sex work plus other work (less economically dependent) |
238
(58.5) 169
(41.5) |
|
Baseline
method of menstrual management Disposable pads Reusable Pads Cloth Cotton Wool/Balls Tissues Tampons |
378
(92.9) 21
(5.2) 32
(7.9) 139
(34.2) 56
(13.8) 9
(2.2) |
|
Sex work during menses
Stays same
Reduces
Increases |
173
(42.5) 232
(57.0) 2
(0.5) |
Table 9. Adverse events
|
Measure |
N
(%) |
|||||||
|
|
BL N=407 |
6M N=332 |
12M N=332 |
13M1,2, 3 N=284 |
14M1,2, 3 N=274 |
15M1,2, 3 N=284 |
18M N=331 |
24M N=221 |
|
Abuse
and Violence |
|
|
|
|
|
|
|
|
|
Any Physical abuse (composite of 6 questions) |
59
(14.5) |
20 (6.1) |
44 (13.6) |
NA |
NA |
NA |
36 (10.9) |
28 (12.7) |
|
Any
Sexual abuse (composite
of 4 questions) |
23
(5.6) |
6 (1.8) |
11 (3.3) |
NA |
NA |
NA |
10 (3.0) |
12 (5.4) |
|
Raped or forced sex |
4
(1.0) |
0
(0.0) |
0
(0.0) |
NA |
NA |
NA |
0
(0.0) |
2
(0.9) |
|
Any
Emotional or financial abuse
(composite of 2 qs) |
190
(46.6) |
78 (23.6) |
143 (43.1) |
NA |
NA |
NA |
103 (31.1) |
97 (43.9) |
|
Cervicovaginal
injuries4 |
|
|
|
|
|
|
|
|
|
Vaginal laceration |
0
(0.0) |
0 (0.0) |
0
(0.0) |
|
|
|
0
(0.0) |
0 (0.0) |
|
Cervical laceration |
1
(0.0) |
0
(0.0) |
0
(0.0) |
|
|
|
0
(0.0) |
0
(0.0) |
|
Menstrual
toxic shock syndrome |
0
(0.0) |
0 (0.0) |
0
(0.0) |
|
|
|
0
(0.0) |
0 (0.0) |
|
Client was angry you were having your
menses |
52 (12.8) |
24 (7.2) |
29 (8.7) |
|
|
|
1 (0.3) |
0 (0.0) |
|
Client
reaction to menses |
|
|
|
|
|
|
|
|
|
Refused to pay, paid |
25 (6.1) |
10 (3.0) |
10 (3.0) |
|
|
|
0 (0.0) |
0 (0.0) |
|
Verbal assault |
23 (5.6) |
10 (3.0) |
10 (3.0) |
|
|
|
1 (0.3) |
0 (0.0) |
|
Physical assault |
2
(0.6) |
0
(0.0) |
0
(0.0) |
|
|
|
0
(0.0) |
0
(0.0) |
|
Cup related |
|
|
|
|
|
|
|
|
|
Had
menses since |
|
|
|
236 (83.1) |
264
(96.4) |
281
(98.9) |
|
|
|
Received
cup |
|
|
|
|
|
|
279
(84.3) |
194 (87.8) |
|
Still
have cup |
|
|
|
|
|
|
270 (96.8) |
183 (94.3) |
|
Used
cup |
|
|
|
222 (94.1) |
258 (97.7) |
274 (97.5) |
259 (92.8) |
183 (94.3) |
|
Cup retention |
NA |
NA |
NA |
6
(2.7) |
1
(0.5) |
0
(0.0) |
0
(0.0) |
1
(0.6) |
|
Pain during / after cup use |
NA |
NA |
NA |
16 (7.2) |
3 (1.5) |
2 (0.9) |
1 (0.4) |
0 (0.0) |
|
Partner violence due to cup use
(refused to pay, paid less
than agreed, verbal assault, physical assault) |
NA |
NA |
NA |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
0
(0.0) |
Table 10. Outcomes (every 6 months)
|
Outcome |
N
(%) |
|
Primary: BV cumulative prevalence*
Control period
Baseline prevalence
6 months prevalence Proportion with prior BV
12 months prevalence Proportion with prior BV (BL or 6M)
Intervention period
18 months prevalence Proportion with prior BV (BL, 6M, or
12M)
24 months prevalence Proportion with prior BV (BL, 6M,
12M, 18M) |
171
(42.0) 144
(42.9) 71
(49.7) 145
(43.7) 108
(74.5) 135
(40.8) 115
(85.2) 80
(45.7) 61
(76.3) |
|
Secondary:
STI Composite cumulative incidence* Control period Baseline prevalence Prevalence at 12 months Incidence at 12 months Intervention period Prevalence at 24 months Incidence at 24 months |
90
(22.1) 78
(23.4) 71 (21.3) 31
(17.8) 30
(17.2) |
|
Trichomonas vaginalis
Control period
Baseline prevalence
Prevalence at 12 months
Incidence at 12 months
Intervention period
Prevalence at 24 months
Incidence at 24 months |
30
(7.4) 25
(7.5) 25
(7.5) 10
(5.7) 10
(5.7) |
|
Neisseria gonorrhoeae Control period Baseline prevalence Prevalence at 12 months Incidence at 12 months Intervention period Prevalence at 24 months Incidence at 24 months |
22
(5.4) 17
(5.1) 15
(4.5) 10
(5.7) 10
(5.7) |
|
Chlamydia trachomatis
Control period Baseline
prevalence Prevalence at
12 months Incidence at 12 months
Intervention period Prevalence at 24 months Incidence at 24 months |
53
(12.8) 45
(13.6) 40
(12.0) 14
(8.1) 14
(8.1) |
|
Secondary:
CST-I (vs. non-optimal vaginal microbiome) Control period Baseline 6 Months 12 Months Intervention period 18 Months 24 Months |
31
(7.6) 16
(4.8) |
|
Mean Relative abundance (SD) L.
crispatus
Control period
Baseline
6 Months
12 Months
Intervention period
18 Months
24 Months |
7.2
(23.7) 4.8
(18.7) |
Table 11. Other Outcomes (monthly)
|
Unsafe menstrual management practices |
N
(%) |
|||||
|
|
BL |
6M |
12M |
18M |
24M |
|
|
The
last time you had sex: did you insert objects inside vagina for sex during
menses (e.g., Kusunda, cotton wool, tissue, sponge, mattress stuffing, foam)
[q65_b1] |
23
(5.6) |
14 (4.3) |
17
(5.1) |
2 (0.6) |
1
(0.5) |
|
|
In the past 6 months: Used drying or
tightening agents inside vagina for sex during menses (e.g., vinegar, soap,
ice, etc.)
Never
Sometimes
Often
Always
Declined |
261
(64.0) 51
(12.5) 12
(2.9) 71
(18.6) 8
(2.0) |
275
(82.6) 25 (7.5) 18
(5.4) 15
(4.5) 0
(0.0) |
292
(88.0) 17
(5.1) 8
(2.4) 15
(4.5) 0
(0.0) |
322
(97.3) 4
(1.2) 3
(0.9) 2
(0.6) 0
(0.0) |
219
(99.1) 2
(0.9) 0
(0.0) 0
(0.0) 0
(0.0) |
|
|
How
often do you use a cloth, tissue, paper, or cotton to wipe inside your
vagina? [q82] Never Sometimes Often Always Declined |
29
(7.1) 10
(2.5) 55
(13.5) 313
(76.9) 0
(0.0) |
131 (39.5) 21 (6.3) 42 (12.7) 138 (41.6) 0 (0.0) |
28
(8.4) 21
(6.3) 57
(17.2) 226
(68.1) 0
(0.0) |
162 (48.9) 13 (3.9) 41 (12.4) 115 (34.7) 0 (0.0) |
61
(27.6) 22
(10.0) 36
(16.3) 102
(46.2) 0
(0.0) |
|