· Blog · 1 min read
POWWeR Health Study
Background This single-arm trial seeks to evaluate the preliminary efficacy of menstrual cups on non-optimal vaginal microbiome (VMB), BV, and STIs of...
Background
This single-arm trial seeks to evaluate the preliminary efficacy of menstrual cups on non-optimal vaginal microbiome (VMB), BV, and STIs of economically vulnerable women at high risk for STIs and HIV, and to understand safety, acceptability, and implementation needs. The goal of this study is to generate preliminary efficacy signals, identify features to optimize implementation, and adverse event monitoring, using as few participants as statistically necessary
Study Design and Measurements over Time
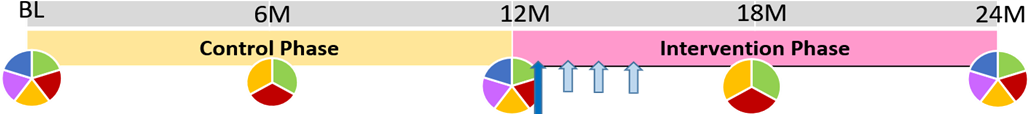
At baseline and every 6-months, we will survey sexual and Menstrual Health Managemengt practices (green), assess VMB (red), and test for BV (orange). We will measure STI (purple) and HIV (blue) at baseline, 12- and 24- months. HSV-2 will be measured at baseline only (not shown in figure). Within 2 weeks of the 12-month visit (blue arrow ), we will conduct menstrual cup training and provide cups to women in groups of 12-15. Telephone survey at 1-, 2-, and 3- months (light blue arrows) will assess menstrual cup use, challenges, and provide trouble shooting. Research staff telephone numbers will be provided should participants require communication between these appointments.
Current Findings